Addressing the challenge of chronic respiratory diseases
By targeting the bacterial biofilm, we aim to relieve the burden of chronic lung conditions such as cystic fibrosis (CF), bronchiectasis, and nontuberculous mycobacterial (NTM) lung disease.
Biofilms are protective barriers formed around colonies of bacteria to shield them from immune effectors or antibiotic intervention. Biofilms are known to drive inflammation and progressive lung damage, or bronchiectasis, which is associated with a range of chronic respiratory conditions.
Bronchiectasis is a condition in which the walls of the lung bronchi become thickened, impairing the ability to clear mucus and trapping bacteria. Over time, this cycle of infection and inflammation can cause progressive damage and dysfunction in the lungs.
CMTX-101: A promising experimental immune-enabling antibody designed to destroy bacterial biofilms by binding to and removing connector proteins within the biofilm structure.
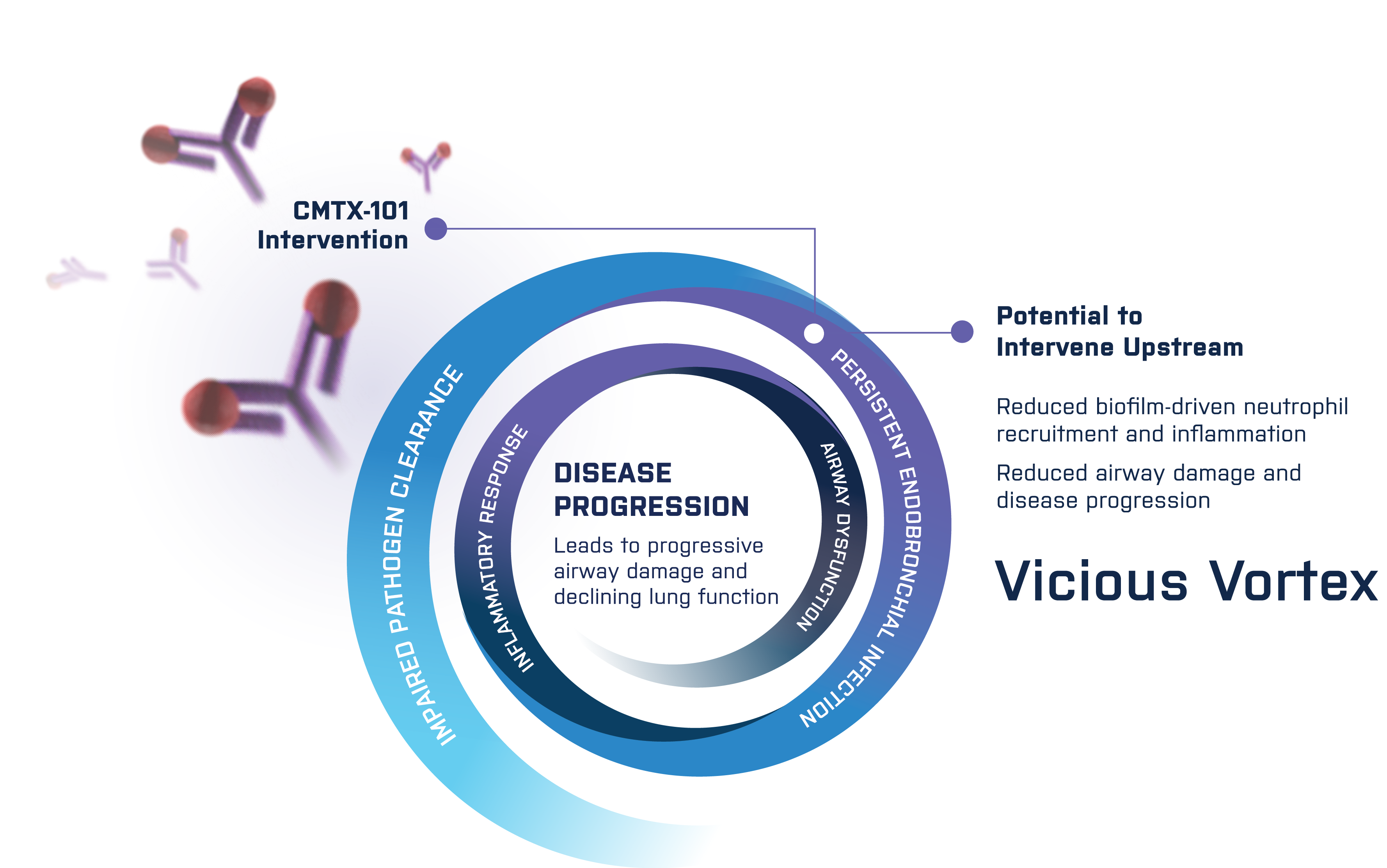
Intervening to Disrupt the "Vicious Vortex" 1,2
Our focus is on the biofilm as a driver of these progressively damaging lung conditions.
Employing CMTX-101 to rapidly dismantle the biofilm may achieve several important outcomes:
- Improve the immune system or antibiotics’ ability to destroy bacteria, thereby reducing inflammation and progressive lung function decline
- Disrupt the cycle of progressive inflammation, damage and dysfunction associated with chronic lung disorders
- In a preventive setting, inhibit the formation or maturation of biofilms to help reduce the incidence and severity of bacterial infections
1.Keir HR, Chalmers Semin Respir Crit Care Med (2021). doi: 10.1055/s-0041-1730891.
2.Flume PA, Chalmers JD, Olivier KN. Lancet (2018). doi: 10.1016/S0140-6736(18)31767-7.
Demonstrating Impact: Ongoing study in cystic fibrosis (CF)-associated infections
CMTX-101is currently being evaluated as part of an ongoing Phase 1b/2a randomized, double-blind, placebo-controlled clinical trial.
- Purpose: Assess CMTX-101 as an adjunctive therapy to standard of care antibiotics in people with CF, evaluating safety and tolerability, pharmacokinetics, immunogenicity, reduction of pulmonary Pseudomonas aeruginosa burden, and additional exploratory endpoints
- Visit our pipeline for more information or visit ClinicalTrials.gov